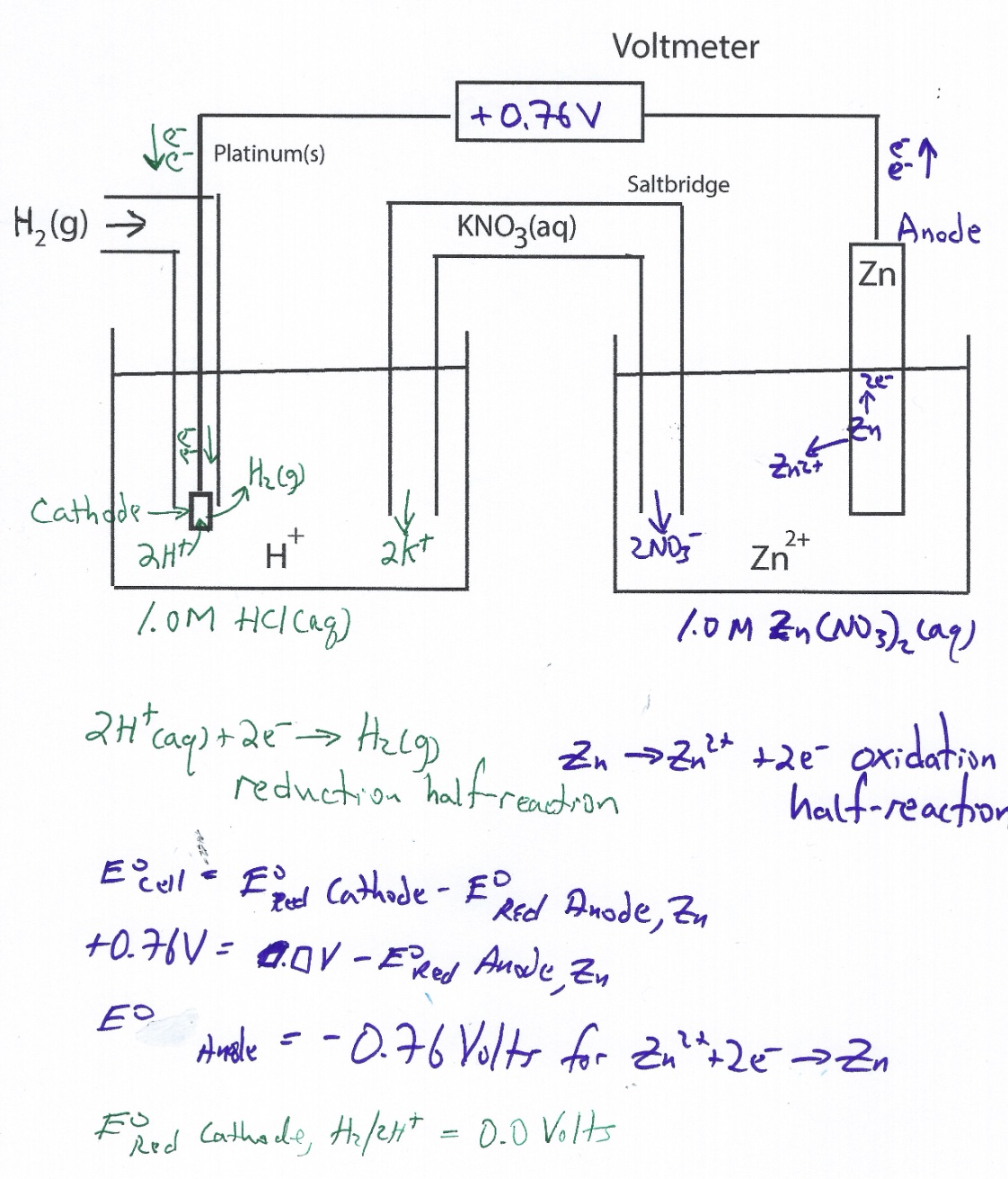
Standard Hydrogen Electrode Zinc . the standard hydrogen electrode is assigned a potential of zero volts. Electrode potential is a measure of reducing power of any element. So, when we close the. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. The second hurdle is overcome by choosing standard. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. It is also called standard reduction. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions.
from cider.uoregon.edu
Electrode potential is a measure of reducing power of any element. the standard hydrogen electrode is assigned a potential of zero volts. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. It is also called standard reduction. So, when we close the. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The second hurdle is overcome by choosing standard. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other.
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER
Standard Hydrogen Electrode Zinc the standard hydrogen electrode is assigned a potential of zero volts. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. Electrode potential is a measure of reducing power of any element. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. the standard hydrogen electrode is assigned a potential of zero volts. So, when we close the. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The second hurdle is overcome by choosing standard. It is also called standard reduction.
From www.researchgate.net
XRD patterns of zinc electrodes after 24h hydrogen evolution test in Standard Hydrogen Electrode Zinc The second hurdle is overcome by choosing standard. Electrode potential is a measure of reducing power of any element. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to. Standard Hydrogen Electrode Zinc.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Zinc So, when we close the. the standard hydrogen electrode is assigned a potential of zero volts. Electrode potential is a measure of reducing power of any element. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. the potential of the standard. Standard Hydrogen Electrode Zinc.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Standard Hydrogen Electrode Zinc 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the standard hydrogen electrode is assigned a potential of zero volts. The second hurdle is overcome by choosing standard. Electrode potential is a measure of reducing power of any element. It is also called standard reduction. . Standard Hydrogen Electrode Zinc.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Zinc The second hurdle is overcome by choosing standard. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. So, when we close the. because zinc has a greater tendency to. Standard Hydrogen Electrode Zinc.
From exovfnkcg.blob.core.windows.net
Zinc Electrode Is Placed In at Conrad French blog Standard Hydrogen Electrode Zinc 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The second hurdle is overcome by choosing standard. the standard hydrogen electrode is assigned a potential of zero volts. So, when we close the. the potential of the standard hydrogen electrode (she) is defined as 0. Standard Hydrogen Electrode Zinc.
From www.youtube.com
standard hydrogen electrode YouTube Standard Hydrogen Electrode Zinc Electrode potential is a measure of reducing power of any element. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. It is also called standard reduction. The second hurdle is overcome by choosing standard. So, when we close the. because zinc has. Standard Hydrogen Electrode Zinc.
From free-images.com
Free Images standard electrode potential zinc Standard Hydrogen Electrode Zinc because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. the standard hydrogen electrode is assigned a potential of zero volts. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard. Standard Hydrogen Electrode Zinc.
From royalweldingwires.com
How Standard Hydrogen Electrode Works Best Guide Standard Hydrogen Electrode Zinc its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. The second hurdle is overcome by choosing standard. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the standard hydrogen. Standard Hydrogen Electrode Zinc.
From slideplayer.com
Electrochemical Cell. ppt download Standard Hydrogen Electrode Zinc 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. So, when we close the. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. Electrode potential is a measure of reducing. Standard Hydrogen Electrode Zinc.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Standard Hydrogen Electrode Zinc its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. So, when we close the. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. The second hurdle is overcome by. Standard Hydrogen Electrode Zinc.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Zinc its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. It is also called standard reduction. Electrode potential is a measure of. Standard Hydrogen Electrode Zinc.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Standard Hydrogen Electrode Zinc the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Electrode potential is a measure of reducing power of any element. It is also called standard reduction. So, when we close the. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the standard hydrogen. Standard Hydrogen Electrode Zinc.
From www.youtube.com
Determination of Standard Electrode potential of 'Zn and Cu Standard Hydrogen Electrode Zinc the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. So, when we close the. Electrode potential is a measure of reducing power of any element. because zinc has a greater tendency to ionize than does hydrogen gas, a higher potential will be established across the zinc electrode. The second hurdle is. Standard Hydrogen Electrode Zinc.
From www.youtube.com
XI Chemistry Ch07 Lec02 (Determination of electrode potential of zinc Standard Hydrogen Electrode Zinc The second hurdle is overcome by choosing standard. Electrode potential is a measure of reducing power of any element. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. because. Standard Hydrogen Electrode Zinc.
From www.researchgate.net
Hydrogen evolution curves of the zinc electrodes in the blank, 1 g l⁻¹ Standard Hydrogen Electrode Zinc The second hurdle is overcome by choosing standard. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the standard hydrogen electrode is assigned a potential of zero volts. . Standard Hydrogen Electrode Zinc.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Zinc So, when we close the. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. the standard hydrogen electrode is assigned a potential of zero volts.. Standard Hydrogen Electrode Zinc.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Zinc 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. It is also called standard reduction. because zinc has a greater. Standard Hydrogen Electrode Zinc.
From www.vrogue.co
Draw A Neat And Well Labelled Diagram Of Standard Hyd vrogue.co Standard Hydrogen Electrode Zinc its absolute electrode potential is estimated to be 4.44 ± 0.02 v[1] at 25 °c, but to form a basis for comparison with all other. The second hurdle is overcome by choosing standard. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. It is also called. Standard Hydrogen Electrode Zinc.